|
Since a DUNS number is required for FDA Compliance, you can obtain one for free by calling the D&B toll free number 1-866-705-5711. Once all information is processed, D&B will issue you a DUNS number at no cost.
An alternative method is to apply using https://importregistration.dnb.com/. The FDA has published a step by step guide on how to apply using this site here: https://importregistration.dnb.com/FDA%20DUNS%20Portal%20User%20Guide.pdf. For further questions or assistance, feel free to contact us. Information Required: • Legal Name • Headquarters name and address for your organization • Doing business as (DBA) or other name by which your organization is commonly known or recognized • Physical Address, City, State and Zip Code • Mailing Address(is separate from Headquarters and/or physical address) • Telephone Number • Contact Name and Title • Number of Employees at your physical location
2 Comments
We are excited to announce Feng-Yu Lee, the President, of Elite BioMedical Consulting, Inc., will be speaking at the 2021 International Conference on Future Healthcare and Economic Development where she will discuss Regulatory Compliance and Clinical Evaluations on Nov. 22nd, 2021 at 2:15pm-2:45pm TW time (UTC +8).
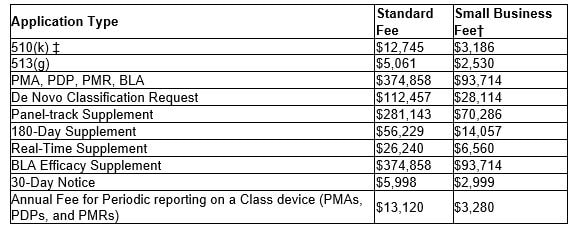
This conference gathers researchers, educators, clinicians and industry leaders around the world engaged in future healthcare research, innovations and issues, and will address the latest trends and perspectives on "Smart Healthcare and Medical Innovation for the New Normal." Both On-Site & Virtual attendance is available and the conference will run from Nov 22-26, 2021. For registration and additional information on the Future Healthcare and Economic Development, click the button link below. We hope to see you there! The following table shows the fees per each medical device submission type for FDA fiscal year 2022, beginning Oct 1, 2021 through September 30, 2022. For eligibility of the reduced small business fee, gross receipts or sales (including sales or receipts from its affiliates) from the most recent tax year must be under $100 million. Also, if a small business has gross receipts or sales of or less than $30 million, they are eligible to have the fee waived for their first PMA, PDP, PMR or BLA. For more information, click the button below to the FDA application or feel free to contact us.
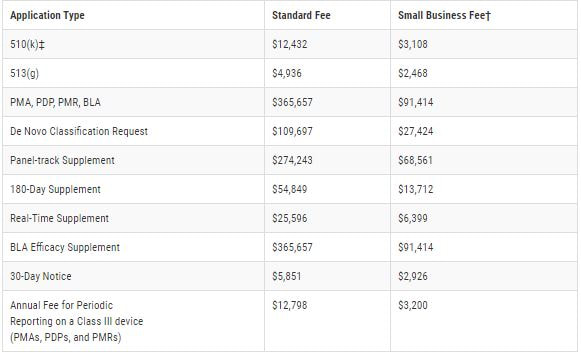
Finally, each establishment is required to be registered with the FDA. The FY 2022 Establishment Registration Fee is $5,672. There are no reductions or discounts for small businesses or establishments and the fee must be paid between October 1, 2021 - December 31, 2021. The following fees per application type, beginning Oct. 1, 2020 through Sept. 30, 2021, are as follows: For eligibility of the reduced small business fee, gross receipts or sales from the most recent tax year must be under $100 million. For additional information, follow the link below to the FDA application or feel free to contact us.
Health Canada has announced amendments to post-market surveillance regulations to all Medical Device License holders and Medical Device Establishment License holders.
The following provisions will come into effect on June 23, 2021: -the power to require that a holder of a medical device licence conduct an assessment (section 62.1) -the power to require that a holder of a medical device licence compile information, conduct tests or studies or monitor experience (62.2) -the requirement of the holder of a medical device authority to notify Health Canada of foreign actions taken in response to a serious risk of injury to human health (sections 61.2 to 61.3) -the requirement to conduct issue-related analyses of safety and effectiveness (section 25(1) and section 39) Additionally, given the new requirement to notify Health Canada of foreign actions taken in response to a serious risk of injury to human health (sections 61.2 to 61.3), the reporting of foreign incidents will no longer be required for class II-IV medical devices as of June 23, 2021. The following provision will come into effect on December 23, 2021: -the requirement of the holder of a medical device licence to prepare summary reports and to notify Health Canada if there has been a change to what is known about the benefits and risks (sections 61.4 to 61.6) For additional details and access to the guidance documents, please check the link below. |
EBMCStay up to date on the latest regulatory news and our latest events & updates. Archives
February 2022
Categories |

 RSS Feed
RSS Feed