|
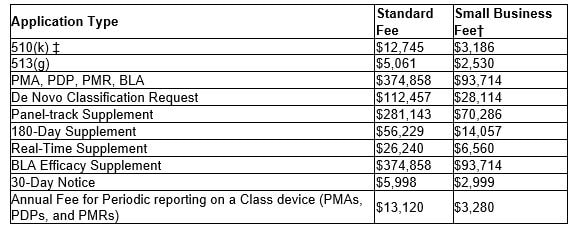
The following table shows the fees per each medical device submission type for FDA fiscal year 2022, beginning Oct 1, 2021 through September 30, 2022. For eligibility of the reduced small business fee, gross receipts or sales (including sales or receipts from its affiliates) from the most recent tax year must be under $100 million. Also, if a small business has gross receipts or sales of or less than $30 million, they are eligible to have the fee waived for their first PMA, PDP, PMR or BLA. For more information, click the button below to the FDA application or feel free to contact us.
Finally, each establishment is required to be registered with the FDA. The FY 2022 Establishment Registration Fee is $5,672. There are no reductions or discounts for small businesses or establishments and the fee must be paid between October 1, 2021 - December 31, 2021.
0 Comments
Leave a Reply. |
EBMCStay up to date on the latest regulatory news and our latest events & updates. Archives
February 2022
Categories |

 RSS Feed
RSS Feed