|
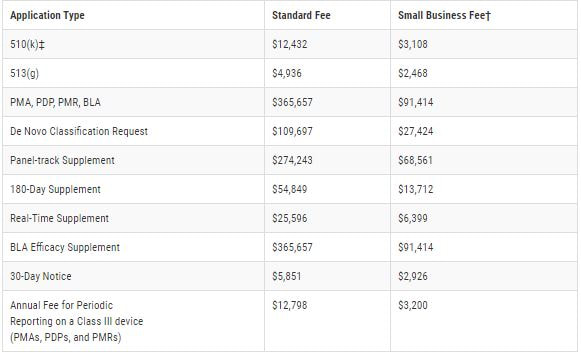
The following fees per application type, beginning Oct. 1, 2020 through Sept. 30, 2021, are as follows: For eligibility of the reduced small business fee, gross receipts or sales from the most recent tax year must be under $100 million. For additional information, follow the link below to the FDA application or feel free to contact us.
1 Comment
6/25/2021 06:56:59 am
Really I enjoy your site with effective and useful information. It is included very nice post with a lot of our resources. Thanks for share. i enjoy this post.
Reply
Leave a Reply. |
EBMCStay up to date on the latest regulatory news and our latest events & updates. Archives
February 2022
Categories |

 RSS Feed
RSS Feed