|
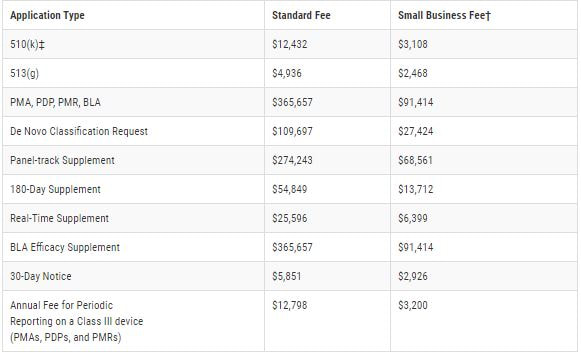
The following fees per application type, beginning Oct. 1, 2020 through Sept. 30, 2021, are as follows: For eligibility of the reduced small business fee, gross receipts or sales from the most recent tax year must be under $100 million. For additional information, follow the link below to the FDA application or feel free to contact us.
1 Comment
Health Canada has announced amendments to post-market surveillance regulations to all Medical Device License holders and Medical Device Establishment License holders.
The following provisions will come into effect on June 23, 2021: -the power to require that a holder of a medical device licence conduct an assessment (section 62.1) -the power to require that a holder of a medical device licence compile information, conduct tests or studies or monitor experience (62.2) -the requirement of the holder of a medical device authority to notify Health Canada of foreign actions taken in response to a serious risk of injury to human health (sections 61.2 to 61.3) -the requirement to conduct issue-related analyses of safety and effectiveness (section 25(1) and section 39) Additionally, given the new requirement to notify Health Canada of foreign actions taken in response to a serious risk of injury to human health (sections 61.2 to 61.3), the reporting of foreign incidents will no longer be required for class II-IV medical devices as of June 23, 2021. The following provision will come into effect on December 23, 2021: -the requirement of the holder of a medical device licence to prepare summary reports and to notify Health Canada if there has been a change to what is known about the benefits and risks (sections 61.4 to 61.6) For additional details and access to the guidance documents, please check the link below. |
EBMCStay up to date on the latest regulatory news and our latest events & updates. Archives
February 2022
Categories |

 RSS Feed
RSS Feed